The ongoing need for healthcare facilities to expand services, renovate patient spaces and improve technologies provides a significant challenge when it comes to protecting patients. Controlling dust, moulds and other pathogens is at the centre of infection prevention measures. However, some of the most threatening contaminants are packaged into ultra-fine particles that are invisible to the naked-eye, are easily disturbed and are difficult to control.
Measuring just 0.005-12 microns in diameter, fine and ultra-fine particles travel via air flow through a facility and are disturbed by construction, renovation or general maintenance activities. These tiny particles pose an increased health risk compared with larger ones because they are small enough to bypass the body’s physical barriers when inhaled; these particles can reside in the lungs or enter the bloodstream. Furthermore, ultra-fine particles are especially dangerous to those who have compromised immune systems from infections or medical treatments.
To help its healthcare clients prevent hospital acquired infections (HAI), preserve reputations and adhere to new government requirements, AMI Environmental is using a multifaceted approach to control dust, validate control measures, and disinfect and commission healthcare spaces.
It is well documented that construction, renovation and maintenance activities generate significant amounts of dust and can disturb contaminants, which travel via the air flow. Therefore, AMI’s priority for any project is to make sure that dust is controlled and the correct pressurisation is in place to direct the contaminated air flow away from patient-occupied spaces.
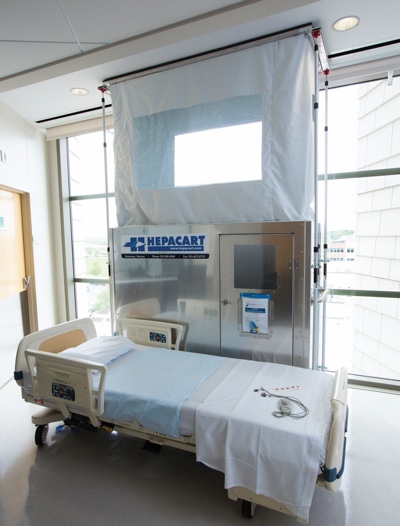
Containment equipment is a requisite for controlling construction-related dust. Hepacart Infection Control Technologies is one of AMI’s go-to partners and has engineered and manufactured suitable equipment for AMI’s projects.
AMI’s equipment might include mobile containment units with high efficiency particulate air (HEPA) filtration for ceiling and wall access, the Hepacart mobile AnteRoom, STARC system wall barriers, negative air machines, HEPA-vacuums and other equipment.
The HEPA-filtration system has been independently lab-tested and proven to effectively collect, filter and contain ultra-fine particles during construction and maintenance work. Each of the Hepacart units is then tested individually to a strict HEPA standard, filtering out 99.99% of contaminants at 0.3 microns.
Tried and tested
AMI Environmental, based in Nebraska (US), recently conducted a case study to determine the effectiveness of Hepacart mobile containment units in controlling project-related dust, during a series of above ceiling work at a Midwest government-owned hospital. Airborne particulate samples were measured before ceiling access, during ceiling access, inside and outside the Hepacart, and after ceiling access was complete.
The Hepacart unit removed 99.99% of pathogens from the above-ceiling air that went through the filtering unit. But the real game-changer came when AMI reviewed particulate measurements outside of the contained space. Data collected in the course of this project consistently showed that in 90% of areas monitored, particulate counts outside the contained space were actually lower after the work was concluded than before work began.
The combination of the negative-filtered containment unit and built-in HEPA filtration pulled and “scrubbed” air from outside the containment unit, delivering extra clean air back into the space during construction work.

In this case study, airborne particulate levels were reduced on average by 26% across all work areas, between initial ceiling access and the completion of work.
Recently, Hepacart has launched a secondary level of air disinfection, which destroys pathogens that are <0.3 microns and may potentially escape the HEPA filter: the Airborne Pathogen Disinfection Module. The new product features Far-UV Sterilray photon light energy to physically destroy molecular bonds within bacteria, viruses and spores.
Independent lab tests have confirmed 5 and 6 log reductions of all contaminants in the filtered air, when using the unit. Many hospitals have seen this as a viable substitute to the practice of ducting filtered air outside; especially when it comes to challenging areas where this is not easily done. “This technology is well-tested and unique to the market,” commented Mark Farnsworth, Hepacar VP. “The physical destruction of pathogens <.03 microns in the air at 0.02 seconds provides a very high level of air disinfection and offers further patient protection,” he explains.
Cleaning and maintaining equipment

The Hepacart AnteRoom unit magnetically seals to a doorframe to provide a secondary vestibule to keep dust and pathogens out of patient areas
Of course, even the best equipment needs a programme for usage, cleaning and maintenance. The number one challenge of infection control directors and preventionists, is the ongoing cleaning and disinfecting of the equipment from one work location to another. “There are some contractors and maintenance personnel who do not realise the danger of moving contaminated equipment from one area to another,” says Farnsworth. “This creates significant risks.”
Hepacart recommends an enforced cleaning process with manufacturer and facility-approved disinfection products. In addition, keeping the contained space under negative pressure throughout the process means more dust and debris is captured in the HEPA-filter and less will deposit on hospital surfaces.
If containment measures are not effective, dust and other ultra-fine particles can escape and travel to just about anywhere in the hospital, including high-risk patient areas like ICU’s, operating rooms, etc. Particulate monitoring is AMI’s preferred method for validating containment measures and ensuring that dust and other contaminants are under control. Particulate monitoring provides a second layer of protection for patients and the facility.
During facility work AMI is able to measure how much dust is escaping from construction work areas so that it can resolve containment problems before patient health is put at risk. In the case of a healthcare-associated infections affecting patients, or lawsuit down the road, particulate monitoring data provides liability protection for healthcare owners who can provide evidence that they approached and carried out work in a responsible manner.
This article appeared in the September issue of Cleanroom Technology.
