The risk-averse nature of healthcare can be a barrier to the adoption of innovative products and technologies that could help to transform thousands of lives, an industry expert is claiming.
Bernard Ross, chief executive of Sky Medical Technology, has 20 years senior experience in pharmaceuticals, technology developments and fast-moving consumer goods (FMCG).
We are very risk averse in healthcare and that’s the pull-and-push pressure. How do we drive change, and how innovative do we want to be?
And he cautions that systems need to keep pace with new technologies and resource local adoption or other similarly-game-changing products are going to be overlooked.
The latest technology Sky Medical Technology has managed to break through into the international healthcare market after a three-year programme of research in the UK is OnPulse.
It was originally developed to prevent blood clots after hip surgery, but it has also been found to heal difficult wounds and accelerate recovery after surgery by reducing swelling.
The system is based on a simple observation: stimulating the common peroneal nerve behind the knee causes the calf muscle to contract. This increases blood flow to the heart and that can have a number of benefits.
The National Institute for Health and Care Excellence (NICE) has since evaluated the device, branded as ‘geko’ when used for medical purposes, and has advised the NHS to use it on patients where compression devices are impractical and contra-indicated.
But the process of health system adoption was challenging and is no doubt putting other companies with ground-breaking products off developing and marketing them to the UK medical market.
Each stage of the process can take months and for many businesses that is too long and not worth the risk or the expense
Speaking to BBH about the challenges, Ross said: “What you need when you develop a product is for that product to be used in real-life situations, and that is where the challenge often lies.
“We are naturally very risk averse in healthcare and that’s the pull-and-push pressure. How do we drive change, and how innovative do we want to be?
“In the UK, surgeons, for example, may be involved in research in order to progress their careers, but not adoption. This research does not necessary have to lead to beneficial change or be linked to a health economy benefit.”
geko was the brainchild of two UK clinicians who worked with Ross to develop and market their initial idea.
But getting the products evaluated in a real-life medical situation proved difficult in a healthcare system with limited capacity or resources to try new products; a problem medtech manufacturers are coming up against daily.
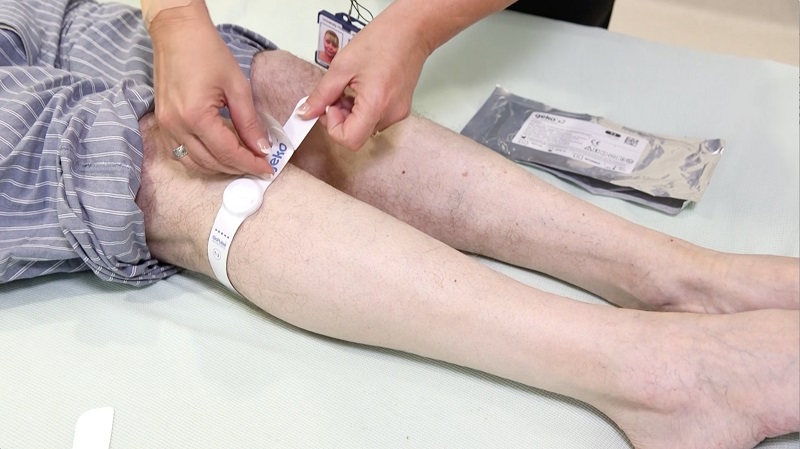
The innovation was trialled at Royal Stoke University Hospital
Ross said: “Learning about new products takes time and money, even when they are NICE recommended as likely to improve patient care.
"When NICE asked trusts to evaluate the device, this required an audit to see if there was a need locally. This process costs money and takes time, so many trusts were wary and NICE was saying they needed to spend this money, so we found ourselves at a stalemate.
“Finally, one trust and one determined clinician in Stoke belived some of his patients were exposed to a high risk of blood clots and felt this product could reduce that risk.
"The trust agreed to carry out a local audit and the result was that they identified a significant unmet need.
“There were stroke patients, for example, who were getting no form of DVT prophylactics. When the device was evaluated on this patient group it showed a significant improvement.”
Ross said: “Other UK trusts have now carried out their own audits and are finding the same level of unmet need and have now adopted the geko device.
Clinicians on the ground understand the most-pressing problems at play, and the UK medtech industry has the means to refine and tailor solutions
“The trouble is that there is limited local emphaisis to resource changes of patient care pathways. For example, it can take months to convene an evaluation committee at each trust and if someone is on holiday, the committee just won’t meet, so you have to wait until they do and that can be months down the line.
"There have even been situations where you can pass every level of local approval, have the product on the shelf in a trust, but then you need a form to be reprinted and and the person in charge of that may say there is no budget for printing new forms.
"The fact that this reprint will save the health system money immediately, and potentially save lives, does not always seem to be understood. We have seen that one issue take more than six months to overcome.
“Each stage of the local adoption process can take months and for many businesses that is too long and not worth the risk or the expense.
“And, while all this is going on, someone could have died.”
He added: “As with many large and complicated organisation there is a siloed mentality in healthcare. These issues are not unique to the NHS as we are seeing the same in other social healthcare systems around the world.
“We need some sort of overriding local authority and we need to be able to prove the benefits of change."
But there is certainly light at the end of the tunnel.
Ross said: “These challenges are understood within the NHS and we are seeing positive changes.
"We are enjoying strong relationships with a rapidly-growing number of clinical partners who are keen to collaborate and get to grips with products to improve the health service.
“We are partnering with teams eager to embrace innovation, generating clinical and health economic data which can be shared across the NHS and internationally.
“Success rests on seamless knowledge transfers between clinicians and industry figures and the resources to make changes at a ward level. Clinicians on the ground understand the most-pressing problems at play, and the UK medtech industry has the means to refine and tailor solutions.”
