BBH speaks to Dr Indira Natarajan, a consultant at the Royal Stoke University Hospital’s acute stroke unit, about the challenges associated with trialling new treatments in stroke aftercare and how embracing innovation and different ways of working can have positive outcomes for patients
Introducing change to clinical practice to address areas of unmet need is complex, both within the NHS and for private healthcare providers.
From ensuring patient safety, to developing a thorough case for investing in new methods or equipment; a number of considerations must be taken into account.
And this was the challenge faced by Dr Indira Natarajan, a consultant stroke physician for the Royal Stoke University Hospital’s acute stroke team, part of the University Hospitals of North Midlands NHS Trust.
Recognising that a number of immobile patients who had suffered ischemic or haemorrhagic strokes were unable to tolerate intermittent pneumatic compression (IPC) to prevent venous thromboembolism (VTE), Dr Natarajan led an observational pilot study to assess a new neuromuscular electrostimulation technology - the geko device - to address this significant unmet need.
As a team, we value remaining at the forefront of innovative treatment and care, and the importance of investing time, effort and determination into improving patient outcomes
And, through close collaboration with the developer of the device, Sky Medical Technology, he has been able to pioneer a change to clinical practice in his unit, to the great benefit of his patients and team.
His work is now driving adoption of the device and change to clinical practice in additional stroke centres in the UK and abroad.
He explains: “Venous thromboembolism (VTE) is a common complication for hospitalised patients, often as a result of restricted mobility.
“It puts patients at risk of potentially-fatal blood clots, and some estimations suggest there are nearly 40,000 deaths in England every year as a result of VTE – over 62% of which could be prevented through proper management and care.”
In particular, acute stroke patients are a highly at-risk population due to reduced mobility, with many patients left bedbound when recovering from the debilitating illness.NICE guidance (NG89) recommend Intermittent pneumatic compression (IPC) as the primary early intervention method to prevent venous stasis in immobile stroke patients.
However, observations from staff at the Royal Stoke University Hospital’s stroke unit, where Dr Natarajan works, suggested that roughly 30% of patients were unable to tolerate, or were contraindicated for, IPC.
Therefore, an alternative anti-stasis intervention was evidently needed to ensure improved outcomes.
Dr Natarajan said: “The Royal Stoke’s stroke unit keeps the betterment of patients at the heart of everything it does.
“As a team, we value remaining at the forefront of innovative treatment and care, and the importance of investing time, effort and determination into improving patient outcomes.
“This was the driving force behind our decision to embark upon an observational pilot study to assess a new VTE preventative method, which could prove a lifeline for at-risk immobile acute stroke patients.”
Current standards of care
One of the challenges facing stroke clinicians is the currently-limited standard of care for post-stroke VTE management, which in the first instance recommends the use of Intermittent Pneumatic Compression (IPC) sleeves.
Not only can these prove uncomfortable for patients, there are a number of contraindications for IPC treatment; some patients present with ulcers or poor blood circulation, making IPC treatment impossible.
There have been times when the team thought we were at the forefront of innovation and it just didn’t work. However, times of failure are times of learning – and it’s better to say you tried and it didn’t work, as you’ve been given the opportunity to think outside of the box
A stroke can also impair cognitive function in patients, who may then remove the sleeves due to discomfort – making it more difficult to monitor patients to ensure they are consistently and correctly wearing them at any one time.
Additionally, while anti-coagulants could be used on a small subset of patients who are contraindicated for IPC, drugs are not recommended by NICE in the first instance due to the possibility of haemorrhagic transformation.

Dr Indira Natarajan
Furthermore, hyper-acute stroke patients are unable to receive drugs at all, so if they also cannot tolerate IPC, a gap in care begins to emerge.
This gap represented an urgent area of unmet clinical need, brought about by the restrictions of only having one non-pharmacological intervention – which limited medics to prevent VTE in every at-risk patient.
The baseline VTE incidence risk of this unmet need in acute stroke patients, reported in the CLOTS3 study, is 8.7% (no IPC, plus standard measures). The study also reports that >30% of acute stroke patients can be contraindicated or cannot tolerate IPC.
Addressing the need
Dr Natarajan said: “As a unit we firmly believe that we must make every effort to provide a standard of care that encompasses the entire patient population.
“This significant unmet need gave us the opportunity to think outside of the box, and explore new ways to address VTE prevention.”
The team met with Sky Medical Technology, a developer of a neuromuscular electrostimulation (NMES) technology. The company was particularly interested in exploring its effectiveness in VTE prevention, and reached out to the stroke unit to discuss collaborative research.
The technology, embedded in a small, wristwatch-style device called the geko, sends painless, regular electrical pulses down a patient’s lower limb, stimulating the common peroneal nerve in the lower leg and activating calf and muscle pumps.
The mechanism of action increases blood flow in the deep veins of the calf – equal to 60% of the blood flow rate of walking, without the patient moving – thereby preventing vascular complications associated with restricted mobility, a common risk for bed-bound stroke patients.
It had clear potential as a non-invasive, non-pharmacological intervention to prevent VTE in a patient group contraindicated or unable to tolerate IPC.
“Of course, all treatment must be evidence based,” said Dr Natarajan.
“As a unit, we recognised the need to generate data to determine geko device efficacy and to remain aligned to our priority of patient safety.
“We agreed to qualify, through an observational pilot study, patient contraindication and intolerance to IPC, to measure geko compliance compared to IPC, and to measure VTE events at 90 days post-stroke.”
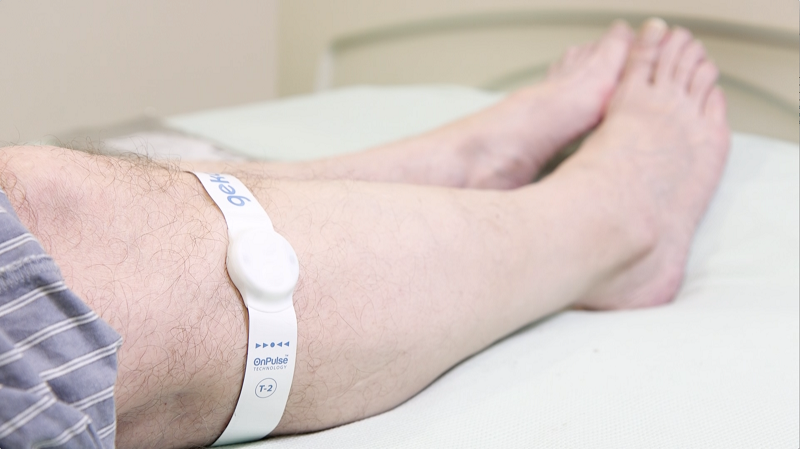
In the study over 90% of patients tolerated the device and there were no incidences of VTE reported 90 days post discharge, compared to 2.4% of patients who received traditional IPC treatment
Changing clinical practice
Dr Natarajan adds: “From my experience of exploring clinical practice change, there can be times of failure.
“There have been times when the team thought we were at the forefront of innovation and it just didn’t work. However, times of failure are times of learning – and it’s better to say you tried and it didn’t work, as you’ve been given the opportunity to think outside of the box.
“This fearless approach was vital when commencing the pilot.”
Any pilot study involving patients involves a huge amount of staff effort, time and investment, not least of all for the initial stages of securing buy-in from the trust.
The close collaboration and support of key stakeholders, including Governance, staff, and most importantly, patients, enabled us to evaluate this new way of preventing VTE
Rigorous processes of governance are required pre-study, and the team first contacted the Directorate Governance for neurosciences to inform them of its wish to commence a pilot observational study and make a solid case for pursuing it.
The study also sought permission from Divisional Governance and Trust Governance;and these groups were on hand to approve each stage of the study, including the proposed inclusion/exclusion criteria and the process of patient recruitment, monitoring and follow up.
It was thanks to the collective effort of the stroke unit team, who ensured the study proposal was properly devised, that Governance agreed permission and support for the research.
Dr Natarajan said: “Given that we were exploring the use of the geko as a prescribed device, we also informed the Royal Stoke University Hospital pharmacy in order to add the geko to a drug chart alongside other standards of care for VTE. This was an important step, which allowed us to implement use of the device in the study. It was also vital to have a database in place to enable the team to track and carefully monitor patients selected to participate.”
With an initial patient goal of 1,000, it took around one year to collect data from each patient.
A study of this scale meant the unit needed additional staffing support and training, and the commitment and buy-in of the entire team to offer their time to making the pilot a success.
Working with Sky, a training programme was put in place to get staff familiar with the device. Additional staff were also enlisted to ensure regular daily checks were carried out with each patient, and participants received regular follow-ups once they had left the unit.
“Having identified a gap in care and the need for change, and having overcome initial apprehensions regarding a potential modification to practice, there was a collective drive to embrace a new, innovative method to ultimately offer improved treatment for all patients – particularly the at-risk cohort,” said Dr Natarajan.
“Introducing any change requires leadership, energy and collaboration. In our case, the close collaboration and support of key stakeholders, including Governance, staff, and most importantly, patients, enabled us to evaluate this new way of preventing VTE.”
Pilot results and patient benefit
Diligent analysis of the initial 1,000 patient data set took just under a year. Of this 1,000 patients, 29.5% were contraindicated or unable to tolerate IPC, making them eligible for an alternative anti-stasis intervention – the geko device.Remaining at the forefront of innovation, carefully gathering evidence, and exploring new alternatives, will benefit our patients and improve treatment in the long term
The data showed that of 463 patients prescribed IPC, 11 patients (2.4%) suffered a VTE event. Of the 203 patients prescribed the geko device, no incidence of VTE was reported 90 days post discharge.
NICE guidance MTG19 supports use of the geko device for people who have a high risk of VTE and for whom pharmacological or other mechanical methods of VTE prevention are impractical or contraindicated. The Royal Stoke team has since implemented NICE guidance when IPC cannot be prescribed.
Over 90% of patients tolerated the geko device, which was favourable when compared to patients prescribed IPC. And compliance feedback was overall very positive, giving reassurance to the unit that the study had been correct and right to pursue.
Dr Natarajan said: “Since sharing these results, our unit has continued with an expansion of the data.
“There are other stroke units across the country also exploring use of the device to prevent venous stasis, and it’s been gratifying to hear from peers in stroke care who recognise the potential for this study and its benefits to patient recovery and outcomes.”
Embracing change and innovation
Commenting on the challenges associated with implementing wisespread change, he added: “Introducing change to clinical practice to address areas of unmet need is complex, especially within the NHS. From ensuring patient safety to developing a thorough case for investing in new methods or equipment, a number of considerations must be taken into account.
“It is by no means an easy undertaking, and it takes considerable investment of staff time, effort and dedication.
If there is major need and, most importantly, if patient benefit is going to be a tangible outcome as a result of change, explore the alternatives to the existing standards of care and embrace innovation
“However, remaining at the forefront of innovation, carefully gathering evidence, and exploring new alternatives, will benefit our patients and improve treatment in the long term.
“To fellow clinicians considering implementing change in their wards and units, I always recommend careful deliberation and consideration of how pressing the issues facing your patients and team are.
“If there is major need and, most importantly, if patient benefit is going to be a tangible outcome as a result of change, explore the alternatives to the existing standards of care and embrace innovation.
“Every clinician keeps patients at the centre of good practice, and ultimately, anything that is good for the patient, their experience, outcomes and benefits, gives a solid platform to move forward with change.”
