Work on a new £2.5m pharmacy and aseptic suite has been completed at Weston Park Hospital.
Designed, engineered, and constructed by BES, the new facilities aim to futureproof the hospital’s drug preparation capabilities on site, with an ATMP-classified environment that enables the production of regenerative medicine, personalised treatments, and the development of nanomedicines.
The project was delivered in two phases to enable the hospital’s existing pharmacy to continue operating on a business-as-usual basis until the new facility was ready for use.
In the first phase, BES refurbished a redundant office area on the sixth floor of the hospital to create the new, high-specification chemotherapy aseptic dispensing facility.
Following commissioning and validation of this facility, the hospital’s pharmacy team moved across from the former pharmacy, vacating that area to allow the phase two refurbishment of the former pharmacy as the new aseptic suite with sophisticated clinical trial (CT) and ATMP preparation facilities.
Working with the hospital’s user requirement specification (URS). and engaging with the hospital’s estates team and senior pharmacist, BES’s multidisciplinary team took the project concept design from RIBA Stage 2 to stage 4, delivering buildability and value engineering benefits.
The facilities were designed to HTM and cGMP requirements and after consultation with the MHRA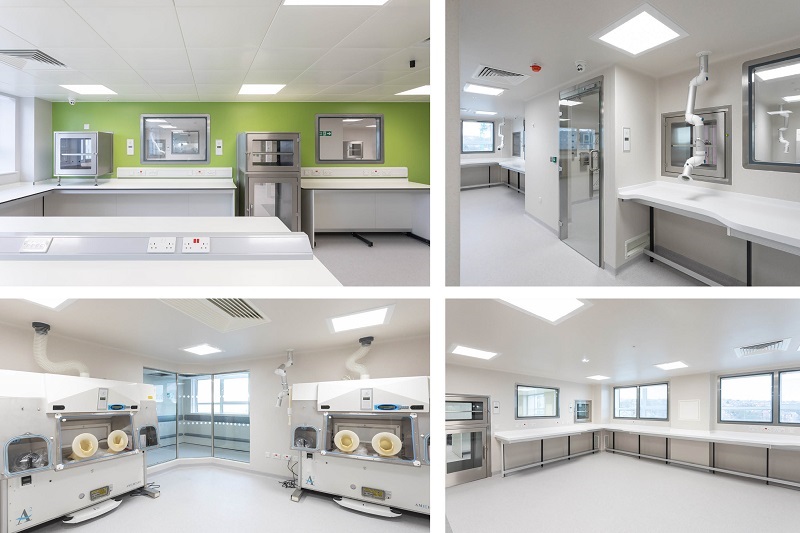
The new facilities were designed to HTM and cGMP pharmaceutical requirements and the design development for the project also involved consultation with the Medicines and Healthcare Regulatory Authority to ensure compliance with regulatory requirements and ensure a smooth validation process.
Delivering the project in BIM, BES designed the facilities using 3D REVIT modelling, along with Navisworks for clash detection.
Lumion software was also used to transfer the model into rendered images to give the client a realistic view of what the facility would look like, and virtual reality walk-throughs allowed the client to experience the facility as part of a collaborative design development process.
Jonathan Morton, the BES engineering director responsible for leading the project, said: “This project leveraged our multi-disciplinary approach to co-ordination of architectural design, building services engineering and construction, along with our collaborative approach to working with the client and end-user stakeholders.
“With responsibility for maintaining business as usual within the live hospital environment and for health and safety as principal designer and principal contractor, we considered the needs of clinicians, service users and patients as an integral part of the design and delivery process.
“The result is a facility that supports the hospital’s reputation for excellence in cancer treatment and creates a template for future gene therapy preparation facilities for other NHS trusts.”
