Asthma UK has released a report highlighting the positive impact connected health can have on the lives of people with the disease.
The document – entitled Smart Asthma: Real-world implementation of connected devices in the UK to reduce asthma attacks – outlines the opportunities for smart inhalers to help improve how asthma care is managed by healthcare professionals, and self managed by people with the condition.
With connected devices expected to be increasingly used in the future, the report also considers how widespread deployment of smart inhalers can be facilitated, and the potential barriers to achieving this.
Without newly-available technologies and the ubiquity of smartphones, significant change would likely be just a pipedream
Smart inhalers can objectively track, monitor, and prompt medication use.
Initial research suggests a willingness among the asthma population to carry a connected device. There is, therefore, a major opportunity for people with asthma, healthcare professionals and the NHS to use data from these devices over the coming decade to improve asthma outcomes in the UK.
However, there could be adverse and costly effects if these technologies are not developed and rolled out thoughtfully.
In the report, Kaye Boycott, charity chief executive, says: “My first major event as chief executive at Asthma UK was the launch of the National Review of Asthma Deaths (NRAD) in 2014.
“This seminal report highlighted the tragedy of two out of three deaths from asthma attacks being entirely preventable if simple routine care was implemented.
“Nobody wants poor care to be the norm. However, we haven’t yet found the best ways of making the basics of asthma management and self management clinically effective, accessible to those without expert knowledge, affordable in a system of stretched resources, and acceptable to the wide variety of people with asthma.
“Continuing with the refrain of ‘if only’ – if only GPs had more time in appointments, if only there was more training, if only patients did exactly what they were told, if only people had a spare five minutes a day – is pointless. We need to design around reality, not aspiration.”
She added: “The reality of managing asthma is that it is messy, unpredictable, complicated, delivered at huge scale, yet entirely personal to each person.
We must have a clear plan to ensure they are safe, effective, and good value for money for all, and to enable rapid access and uptake
“And, crucially, unless someone is struggling for breath, it’s unlikely to be the most important thing on the to-do list of their life.
“These are the underpinning principles for any redesign of how we deliver basic asthma care – solutions need to be discreet, personalised and intuitive, and need to require less from the person with asthma, rather than more.
Without newly-available technologies and the ubiquity of smartphones, significant change would likely be just a pipedream.
“However, with new sensors, cloud data services, risk algorithms, telehealth solutions, and mobile apps we have the opportunity to radically redesign asthma care.”
The report sets out the opportunities and possible pitfalls of digitising asthma services.
It includes feedback from industry, expert clinicians, eminent academics and policy specialists and explores how, to make new technology stick, it must help patients, enhance system efficiency, and allow providers to make a fair profit.
The report states that Smart inhalers have the potential to support:
- Digital transformation of health services
- Management of long-term conditions at scale
- Enhanced supported self management
- Risk stratification and management according to need
- Personalised approaches to care
- Reductions in avoidable emergency admissions
- Improvements in patient safety
- Improvements in patient quality of life
- New discoveries through data-driven academic research
The report states: “Widespread use of connected technologies will happen very soon.
“We must have a clear plan to ensure they are safe, effective, and good value for money for all, and to enable rapid access and uptake.”
It says that to roll out the technology at scale, companies must involve people with asthma in the development of products to encourage greater use.
Clinicians also need to be consulted as they will be key drivers of the technology among their patients, resulting in more-useful and efficacious products, and, potentially, better use of clinicians’ time.
we continue to provide leadership in the respiratory medication adherence space, contributing our extensive research and knowledge base to establish best practice in this key European market
Other observations and recommendations in the report include:
- The technical capability to introduce connected devices to address key issues in asthma care (such as adherence) is already here, though this does not imply that ideal care pathways, processes and devices yet exist
- Realising the potential of connected technologies will require investment in a significant programme of research – including basic information such as accuracy, robustness, user experience, and clinical outcomes
- The promise of connected technologies will not be realised unless substantial work is done on data standards and interoperability with NHS systems
- Clear technical standards for smart inhalers will ensure consumers receive devices that are robust and accurate, and collect clinically-meaningful data. The creation of standards against which organisations like NICE can appraise new products is overdue
- There is currently a lack of clarity over the funding structure for connected devices in the UK and limited understanding of how this will align with developers’ business models. The elucidation of this by NHSE will facilitate innovation and allow healthcare providers to co-ordinate their plans for future services to maximise progress and minimise waste
- The health technology appraisal system across the UK needs to be efficient to ensure that it is an attractive first market destination for smart inhaler companies looking to introduce their products
- An industrial grand challenge for asthma management could seek to harness device and drug-based monitoring technologies, combined with patient data, aimed at automating asthma care where this could be appropriate
- Researchers will need access to data-enabled populations at sufficient size and representation to enable cost-effectiveness testing and development of implementation models for smart inhalers
The report has been welcomed by industry.
Speaking to BBH Garth Sutherland, chief executive of digital health technology manufactuer, Adherium, which contributed to the report, said: “This report highlights some important issues and makes key recommendations to improve the use of and infrastructure around connected devices for asthma management in the UK.
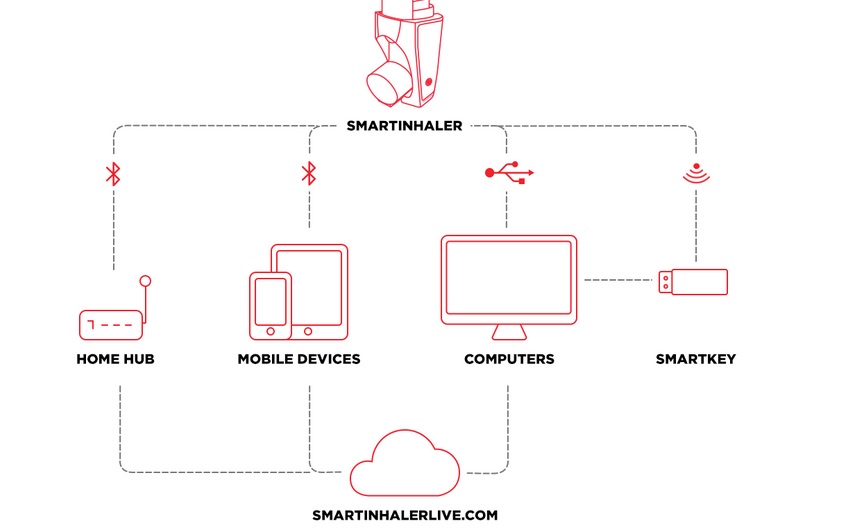
Adherium's The Smartinhaler technology is an example of how these devices can have a positive impact on the condition
“Following NICE’s acknowledgement in its Medtech Innovation Briefing last month that our proprietary Smartinhaler technology can improve adherence to asthma medication, we believe that professionally-developed, clinically-proven devices will be essential in the wider adoption of connected asthma management in future.
We continue to provide leadership in the respiratory medication adherence space, contributing our extensive research and knowledge base to establish best practice in this key European market
“Through our work with Asthma UK and Europe-wide initiatives such as the myAirCoach programme, we continue to provide leadership in the respiratory medication adherence space, contributing our extensive research and knowledge base to establish best practice in this key European market.”
The Smartinhaler technology has been used in more than 65 studies to date and referenced in 56 peer-reviewed journal articles.
Clinical outcomes data has proven that the platform can improve adherence by up to 59% in adults and 180% in children and reduce severe episodes by 60% in adults, leading to improved quality of life and demonstrating a substantial gain over current best practice treatment.
